Prof. Zvi Fishelson, Ph.D.
Department of Cell and Developmental Biology
Research
Prof. Zvi Fishelson, Ph.D.
Department of Cell and Developmental Biology

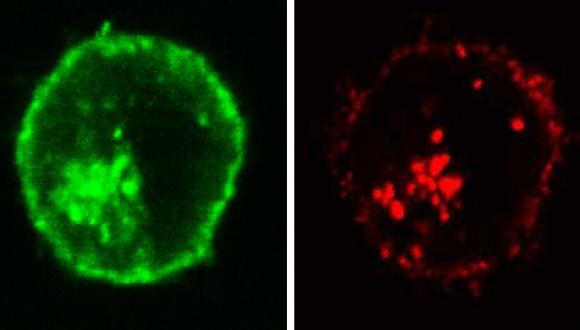
The long-term goal of our research is to develop a novel treatment for immune resistant cancers. Our research includes characterization of the mechanism of complement dependent cytotoxicity and of the basis for elevated resistance of cancer cells to cell death, and design of novel reagents that sensitize cancer cells to cell death. Research methods used include analyses of cell growth and death and mitochondrial activity, western blotting, enzyme-linked immunosorbent assay (ELISA), immunoprecipitation, confocal fluorescence microscopy, Fluorescence-activated Cell Sorting (FACS), peptide analysis by mass spectrometry, electon microscopy, and analysis of cancer growth in animal models.
Research
Prof. Neta Erez, Ph.D.
Department of Pathology

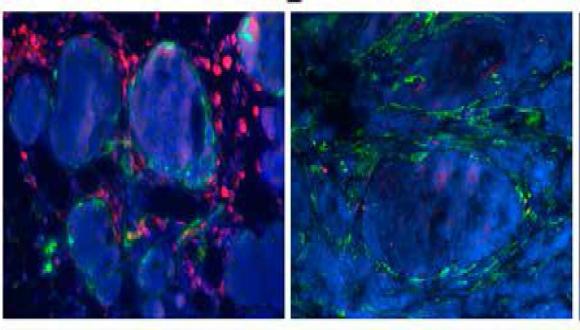
The main goal of our laboratory is to uncover stromal pathways that contribute to tumorigenesis and metastasis. In particular, we combine transgenic mouse models of cancer as well as clinical data to study the role of inflammation and cancer-associated fibroblasts in facilitating lung metastasis of breast cancer, and to uncover the role of neuroinflammation mediated by astrocytes in melanoma brain metastasis.